



There are about 27.8 million people with hearing disabilities in China, accounting for 33%of the total number of people with disabilities, and the proportion of newborndeafness is about 1-3%. More than 30,000 new deaf children are born each year, with more than 60%of the genetic factors causing 3 deafness. Environmental factors (about 20%),unknown factors (about 20%),in the normalpopulation, deaf gene mutation carriers more than 80 million people. A large number of patients with late hearing loss are deaf engenedeaf by their own genetic defects, or by genetic defects and polymorphism, resulting in sensitivity to deafening environmental factors, and thus disease-causing. The traditional detection method can not detect the deafness caused by late deafness or gene mutation in time, and the testing of deafness can play the effect of early diagnosis, early detection and early intervention.
High sensitivity:the detection limit of the genomic DNA of the examinee is 2ng / μL;
High accuracy: verified by 5000 multi-center clinical samples, the conformity rate with sequencing is 100%;
Authoritative institution clinical verification:

|
Obstetrics, Neonatal |
Gynecologic |
Otolaryngology |
Internal Medicine, Surgery |
|
Neonatal |
Pre-pregnancy, early pregnancy women |
Hearing-impaired patients and their families |
Users of amino glycoside drugs |
|
Early detection of congenital deafness, late-onstage deafness and drug deafness, early diagnosis, early prevention, early intervention |
Screening carriers of deaf engene mutations to provide genetic counselling and guidance for birth defects |
Hearing-impaired family marriage guidance and medication guidance, prediction of cochlear implant effect |
Guidance on the use of antibiotics in aminoglycoside drugs to prevent drug deafness |
Normal (N/N)
|
35N● |
176N● |
235N● |
299N● |
538N● |
1494N● |
1555N● |
IVS7-2N● |
1226/1229N● |
2162/2168N● |
Number |
|
35M |
176M |
235M |
299M |
538M |
1494M |
1555M |
IVS7-2M |
1226M |
2168M |
|
|
167M |
281M |
589M |
IVS15+5M |
547M |
1975M |
2027M |
1174M |
1229M |
2162M |
Single-mutation pure hejuno(IVS7-2M)
|
35N● |
176N● |
235N● |
299N● |
538N● |
1494N● |
1555N● |
IVS7-2N |
1226/1229N● |
2162/2168N● |
Number |
|
35M |
176M |
235M |
299M |
538M |
1494M |
1555M |
IVS7-2M● |
1226M |
2168M |
|
|
167M |
281M |
589M |
IVS15+5M |
547M |
1975M |
2027M |
1174M |
1229M |
2162M |
Single Mutant Heoco(35M/N)
|
35N● |
176N● |
235N● |
299N● |
538N● |
1494N● |
1555N● |
IVS7-2N● |
1226/1229N● |
2162/2168N● |
Number |
|
35M● |
176M |
235M |
299M |
538M |
1494M |
1555M |
IVS7-2M |
1226M |
2168M |
|
|
167M |
281M |
589M |
IVS15+5M |
547M |
1975M |
2027M |
1174M |
1229M |
2162M |
Test specimen: Anticoagulant whole blood sample
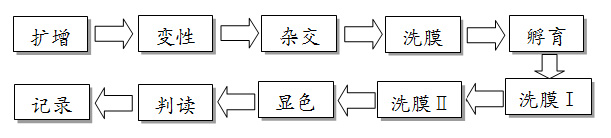
Technical principle:PCR - reverse point hybridization
Packing size:25 test / kit
Class: In vitro diagnostic reagents
Applicable instruments: common gene amplification instrument, molecular hybridizer