


Glucose-6-phosphate dehydrogenase(G6PD) deficiency is one of the most common X-chain incomplete genetic diseases in the world, commonly known as broad bean disease. Clinically, it is manifested as neonatal jaundice, broad bean disease, drug-based hemolytic, infectious hemolytic and non-spherical cell hemolytic anemia and other diseases. In children and infants concentrated in the performance of broad bean disease and neonatal jaundice, often more serious, if not timely treatment will endanger the lives of children. About 200 million people worldwide are affected. About 50% of newborns with G6PD deficiency develop neonatal jaundice, and about 12% of them develop nuclear jaundice, leading to brain damage and low intelligence.
|
1376N |
1388N● |
487N● |
95N● |
392N● |
871N● |
592N ● |
|
1376M● |
1388M |
487M |
95M |
392M |
871M |
1004M● |
|
1381M |
1387M |
493M |
592M |
1004M |
1024M |
1024M● |
A: Genetic testing, the results are accurate and reliable for life.
B: For patients with G6PD gene mutation, clear and targeted medication guidance can be given to avoid contact with drugs that may cause acute hemolysis.
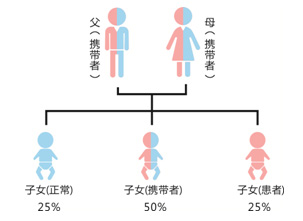
C: Genetic testing can effectively detect female heterozygotes and neonatal hemolysis.
D: Definite family genetic history. Provide guidance on marriage and childbirth and antibiotic medication.
E: Early detection of G6PD deficiency caused by genetic factors. Newborns can start preventive interventions from their mothers.
|
Comparison items |
Chemical methods |
Melting curve method |
Glass chip method |
PCR Reverse Point Hybridization (Billion Cube) |
|
Detection accuracy |
Unable to detect female hybrids, affected by environmental impact |
Small differences in mutation temperature of adjacent sites can lead to miscalculation of results |
Chip hybrid conditions are difficult to unify, and specificity needs to be improved |
More than 99.9% of conformity with sequencing control results |
|
Detecting sites |
No-type |
12 sites |
7 sites (including 1 non-pathogenic site) |
12 sites |
|
Instrument |
Biochemical instrument |
Fluorescence quantitative PCR instrument |
Requires dedicated equipment |
Common hybrids |
G6PD deficiency genotyping detection
Risk assessment of G6PD deficiency in child care before marriage examination
Prenatal G6PD deficiency screening high-risk groups, newborn screening
G6PD gene mutation population to check allergies before using antibiotics
Test specimen:Anticoagulant whole blood sample
Technical principle:PCR - reverse point hybridization
Packing size:25 tests / kit
Class:In vitro diagnostic reagents
Suitable instruments:Common gene amplification instrument, molecular hybridizer