


Epstein-Barr virus (EBV) is a member of the genus Lymphotropic Virus of the Herpesviridae family, and its genome is DNA. Epstein-Barr virus has the biological characteristics of specifically infecting humans and certain primate B cells in vivo and in vitro. Humans are hosts of Epstein-Barr virus infection, spread mainly through saliva. 90% of adults worldwide are positive for EBV antibodies. The first infections in Asia and developing countries often occur in children, and the first infections in the United States and Western Europe often occur in adults. Diseases related to Epstein-Barr virus include infectious mononucleosis, hemophagocytic syndrome, chronic active Epstein-Barr virus infection, X-linked lymphoproliferative disease, etc. In particular, it is worth noting that EB virus is an important tumor-related virus, which is closely related to the occurrence of various tumors such as nasopharyngeal cancer, lymphoma, and gastric cancer. The number of people affected by EB virus-related tumors in the world reaches 1%.
Studies have shown that the positive rate of EBV VCA-IgG antibody in children aged 3 to 5 years in China is more than 90%, and most children have no obvious symptoms after infection, or cause mild pharyngitis and upper respiratory tract infection. Primary infections occur in adolescence, and about 50% of them develop infectious mononucleosis. It is mainly spread through saliva and can also be transmitted through blood transfusions.
|
EB virus nucleic acid detection kit (PCR-fluorescent probe method) |
|
|
Sensitivity |
5.0x103copies/mL |
|
Linear range |
1.0x104copies/mL ~ 1.0x 109copies/mL |
|
Accuracy |
With reference to international reference products, the coincidence rate of the test results is 100% |
|
Precision |
Coefficient of variation within and between batches CV≤5% |
|
Specificity |
100% specificity, no cross reaction with Streptococcus pneumoniae, Mycobacterium tuberculosis, Mycoplasma pneumoniae, Pertussis bacteria, adenovirus, respiratory syncytial virus, etc. |
|
Anti-interference |
Mucin, pus, erythromycin, chloramphenicol did not interfere with the test results |
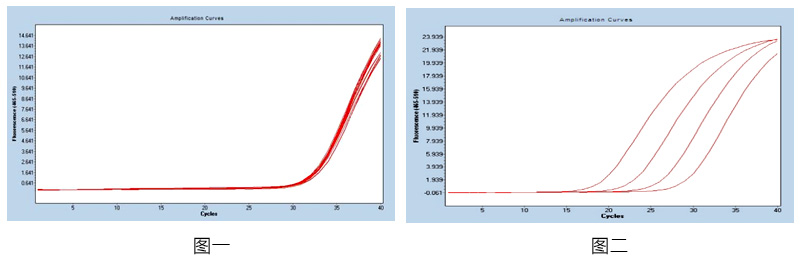
Figure 1: Using 1x104copies / mL reference as a template, repeat the 20-well test in the same batch, calculate the coefficient of variation of the CT value in the batch, and the CV value is ≤5%, with good consistency. product information
Figure 2: For the reference products in the concentration range of 107-104 copies / mL, the product amplification curve is smooth and complete, and the correlation coefficient between different gradients is R> 99%, and the correlation is good.